Catalyst-Directing Groups in Organic Synthesis and Catalysis
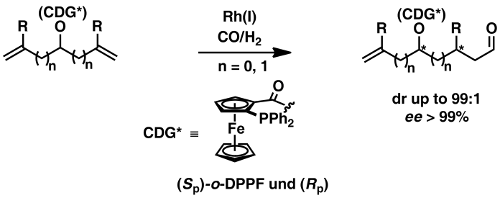
In order to develop any chemical reaction into a useful and efficient synthetic method the issue of selectivity control has to be solved. For this reason we have developed the rational selectivity control instrument for a catalytic reaction, which is the use of substrate-bound catalyst-directing groups (CDG).[38,47,49] These groups function as secondary binding posts of a substrate, which allows a reversible multipoint attachment of the substrate to the catalyst. The result is a highly ordered cyclic or polycyclic transition state, which ensures control of any type of selectivity. Following this idea we were able to control regio- and stereoselectivity in a number of catalytic and metalorganic reactions (hydroformylation of alkenes,[49,52,81,89] cuprate addition, [26,37] copper-catalyzed allylic substitution [44,56,72,73,76,105,113,114,119] see Scheme 1).
Scheme 1: Selective catalytic carbon-carbon bond forming reactions with the aid of Catalyst-Directing Groups (CDG).
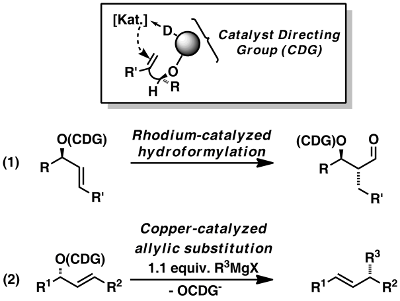
A recent development is the use of chiral CDG*’s [55,59,63-65,108,109] which allow for the first desymmetrizing hydroformylation of bisalkenyl and bisallyl carbinols (Scheme 2). The corresponding monoaldehydes were formed with excellent diastereotopic face- and perfect diastereotopic group discrimination in enantiomerically pure form.
Scheme 2: Desymmetrizing hydroformylation with the aid of a chiral Catalyst-Directing Group (CDG*).