Zinc-Catalyzed Enantiospecific sp3-sp3 Cross-Coupling of α-Hydroxy Ester Triflates with Grignard Reagents
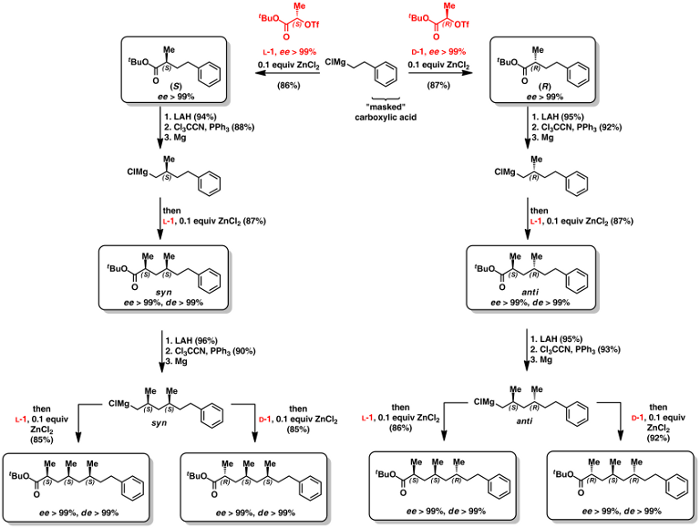
The construction of α-chiral carbonyl compounds is traditionally achieved via enolate alkylation employing chiral auxiliaries. The drawbacks of this approach are the costs and problems with recovery of the auxiliary and the problems with reactivity employing sterically demanding substrates. To circumvent these problems we have developed a zinc-catalyzed enantiospecific cross coupling reaction of α-hydroxy triflates with Grignard reagents (Scheme 9).[104] A number of α-hydroxyacids and esters are availabe from the chiral pool or are readily prepared from either α-amino acids or enzymatically generated cyanohydrins. Thus, transformation of the hydroxy group into a triflate leaving group allowed for an enantiospecific sp3-sp3 cross coupling reaction with Grignard reagents in the presence of zinc dichloride as the catalyst.
Scheme 9: Zinc-catalyzed enantiospecific cross coupling reaction of α-hydroxy triflates with Grignard reagents.[104]
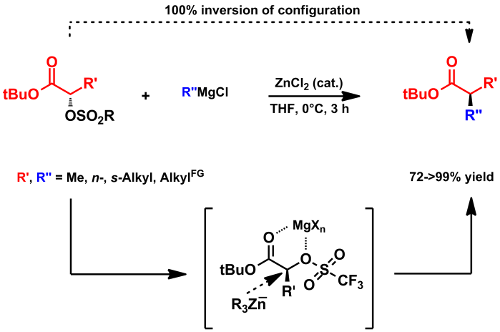
The methodology could be used as the basis for a new iterative strategy for the stereospecific construction of deoxypropionate building blocks (Scheme 10).[117]
Scheme 10: Zinc-catalyzed enantiospecific cross coupling as the key step for an iterative construction of deoxyproionates.[117]