A Unified Strategy for the Stereospecific Construction of Propionates and Acetate-Propionates Relying on a Directed Allylic Substitution
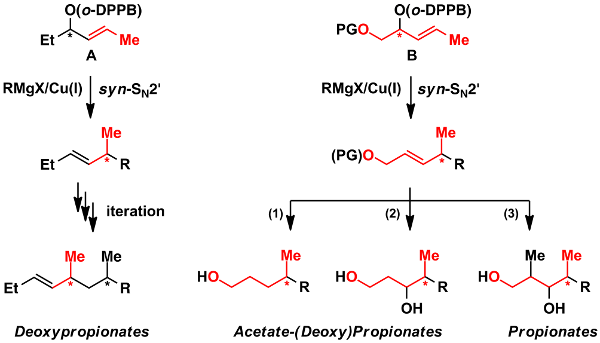
Propionate and deoxypropionate structural elements are found in a number of biologically relevant and medicinally interesting natural products. We recently devised a new strategy for the enantioselective construction of such elements employing our copper-catalyzed directed allylic substitution. It is thus an alternative to traditional aldol and enolate alkylation methodology with reversal of polarity (Scheme 6).[57,73,113]
Scheme 5: A new iterative synthetic strategy for the flexible construction of deoxypropionates.
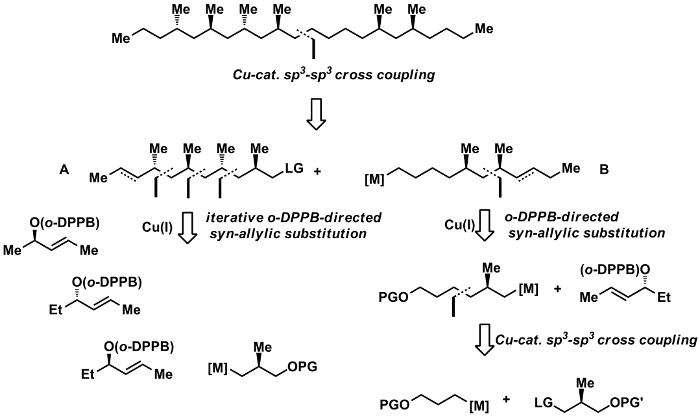
The methodology has been successfully applied in a number of natural product synthesis.[62,83,87,90,113,114,119] Thus, total synthesis of a pheromone of the Australian sugar cane beetle has allowed to establish its absolute configuration (Scheme 7).[62,83]
Scheme 7: Enantioselective Total Synthesis and Determination of the Absolute Configuration of the 4,6,8,10,16,18-Hexamethyldocosane from Antitrogus parvulus.[62,83]
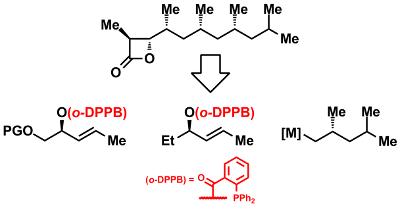
Combining deoxypropionate and propionate synthesis enabled an efficient total synthesis of vittatalactone. This allowed to establish the relative and absolute configuration of this important pheromone of the striped cucumber beetle (Scheme 8).[119]
Scheme 8: Enantioselective Total Synthesis and Determination of the Absolute Configuration of Vittatalactone.[119]
Currently the methodology is applied to the construction of the angiogenese inhibitor borrelidine and TMC-151 showing antitumor activity.

