N E W S
Congratulations to Our Research Groups Running Team
On April 8, 2024, a team from our research group (and Tom from the Schnitzer Lab) successfully took part in the Freiburg Marathon as a relay team. We congratulate our runners on their impressive twentieth place with a very good time of 3:25:47 for the 42.2 km.
New Publication in Accounts of Chemical Research
"Rhodium-Catalyzed Allylic Addition as an Atom-Efficient Approach in Total Synthesis"
Finding efficient synthetic methods for the asymmetric synthesis of complex molecules has always been of interest to organic chemists. Creating and controlling the stereochemistry of stereogenic centers bearing branched allylic moieties in organic molecules using a catalytic process is an attractive and successful method for the synthesis of several natural products and medicinally important compounds. Remarkable progress toward their synthesis has been achieved via transition-metal catalysis, especially in the case of allylic substitution and allylic C–H oxidation chemistry. However, for allylic substitution the preinstallation of a leaving group is essential, and for allylic C–H oxidation, stoichiometric amounts of oxidant are required. Besides that, the control of regioselectivity with these methods is often problematic because the linear product can be produced as a major isomer. Our research group has developed a regioselective, enantioselective, and atom economic route toward the more valuable branched product via a Rh-catalyzed coupling of easily accessible alkynes or the double-bond isomeric allenes with pronucleophiles. It was demonstrated that, using this new approach, it is possible to add different pronucleophiles to alkynes or allenes to form branched allylic moieties through C–C and C–heteroatom bond formation. Since new organic reactions offer new opportunities in chemical synthesis and the benchmark for new synthetic methods is their application in target-oriented synthesis, we have demonstrated several successful syntheses of natural products and medicinally relevant targets. For example, in the total syntheses of Quercuslactones, Helicascolides A–C, Epothilone D, Homolargazole, and Thailandepsin B, the Rh-catalyzed hydro-oxycarbonylation of allenes was used as key step via C–O bond formation. Remarkably, the Rh-catalyzed C2-symmetric dimerization strategy was used to synthesize the complex molecules Clavosolide A and Vermiculine, leading to an extreme increase in structural complexity within a single step. For the total syntheses of Centrolobine, Pitavastatin, and Rosuvastatin, C–O bond formation was achieved through the addition of a hydroxy function to the allene moiety. The potential of the addition of nitrogen pronucleophiles to allenes was demonstrated in the total syntheses of Cusparein, Angusterein, Cermicin C, Senepodin G, Homoproline, Pipecolinol, Coniceine, Coniine, Ruxolitinib, Sitagliptin, Abacavir, Glucokinase activators, and Chaetominine. All of these examples testify to the wide applicability of the Rh-catalyzed addition of pronucleophiles to allenes or alkynes in target-oriented synthesis, and in this Account we summarize our contribution.
F. Panahi, F. Bauer, B. Breit, Acc. Chem. Res. 2023, 56, 24, 3676–3693. (link)
Bachelor Topics Organic Chemistry Summer 2024
The research groups of the Organic Chemistry Department will present the bachelor topics for the next summer semester on 11.01.2024 at 17:15 in the HS Chemie.
https://ilias.uni-freiburg.de/
Congratulations to Günter on his 40th anniversary of service!
We thank Günter for his loyalty and dedication. We look forward to working with him in the future and wish him all the best.
| |
Bachelor Topics Summer 2023
We would like to invite all interested students to find the topics offered by our research group for a bachelor thesis at the following link on the ILIAS platform
https://ilias.uni-freiburg.de/
Congratulations to Dr. Wolfgang Seiche on his 25th anniversary of service in
our research group!
Congratulations to Dr. Wolfgang Seiche on his 25th anniversary of service in our research group! For 25 years, Wolfgang has done excellent work and contributed greatly to the success of our group.
As a chemist, Wolfgang has initiated the topic of self-assembly ligand as his most outstanding research contribution. In addition, Wolfgang has also been an important organizer within the group. His ability to keep the group running smoothly and efficiently has been vital to our success.
We thank Wolfgang for his loyalty and dedication and look forward to working with him in the future. On this occasion, we congratulate him on his anniversary of service and wish him all the best for the future.
| |
Soccer Team of Breit Group wins Husemann-Cup for the first time
On September 22, 2022, the soccer team of the Breit group won the prestigious Husemann Cup for the first time. It is especially remarkable that for the first time a team from the Institut für Organische Chemie could win this tournament. The entire working group congratulates its team on this great success!
New Publication in Angewandte Chemie
Regio-, Diastereo-, and Enantioselective Decarboxylative Hydro-aminoalkylation of Dienol Ethers Enabled by Dual Palladium/Pho-toredox Catalysis
Intermolecular photocatalytic hydroaminoalkylation (HAA) of alkenes have emerged as a powerful method for the construction of alkyl amines. Although there are some studies aiming at stereoselective photocatalytic HAA reactions, the alkenes are limited to electrophilic alkenes. Herein, we report a highly regio-, diastereo-, and enantioselective HAA of electron-rich dienol ethers and α-amino radicals derived from α-amino acids using a unified photoredox and palladium catalytic system. This decarboxylative 1,2-Markovnikov addition enables the construction of vicinal amino tertiary ethers with high levels of regio- (up to >19 : 1 rr), diastereo- (up to >19 : 1 dr), and enantioselectivity control (up to >99 % ee). Mechanistic studies support a reversible hydropalladation as a key step.
J. Zheng, N. Tang, X.Hui, B. Breit Angew. Chem. 2022, ASAP ; (link) Angew. Chem. Int. Ed. 2022, ASAP. (link)
Looking for a position? We are hiring!
Highly motivated and talented bachelor, master and PhD students welcome to join our group.
New Publication in Chemical Science
Rhodium-catalyzed asymmetric intramolecular hydroarylation of allenes: access to functionalized benzocycles
A rhodium-catalyzed regio- and enantioselective cyclization of tethered allenylbenzenes is reported. Employing a RhI/Josiphos catalytic system a diverse set of 6-membered, α-chiral benzocycles were obtained, scaffolds found in many bioactive compounds. Moreover, a gram scale reaction as well as the application of suitable transformations are demonstrated.
D. Berthold, J. Klett, B. Breit, Chem. Sci. 2019, 10, 10048-10052. (link)
New Publication in Nature Communications
A domino reaction for generating β-aryl aldehydes from alkynes by substrate recognition catalysis
The development of universal catalyst systems that enable efficient, selective, and straightforward chemical transformations is of immense scientific importance. Here we develop a domino process comprising three consecutive reaction steps based on the strategy of supramolecular substrate recognition. This approach provides valuable β-aryl aldehydes from readily accessible α-alkynoic acids and arenes under mild reaction conditions, employing a supramolecular Rh catalyst containing an acylguanidine-bearing phosphine ligand. Furthermore, the synthesis of a key intermediate of Avitriptan using this protocol is accomplished. The first step of the reaction sequence is proved to be the regioselective hydroformylation of α-alkynoic acids. Remarkably, molecular recognition of the ligand and the substrate via hydrogen bonding plays a key role in this step. Control experiments indicate that the reaction further proceeds via 1,4-addition of an arene nucleophile to the unsaturated aldehyde intermediate and subsequent decarboxylation.
W. Fang, F. Bauer, Y. Dong, B. Breit, Nat. Commun. 2019, 10, 4868. (link)
New Publication in Angewandte Chemie
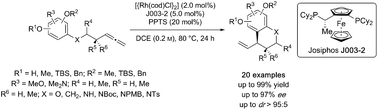
Rhodium-Catalyzed Asymmetric Intramolecular Hydroamination of Allenes
The rhodium‐catalyzed asymmetric intramolecular hydroamination of sulfonyl amides with terminal allenes is reported. It provides selective access to 5‐ and 6‐membered N‐heterocycles, scaffolds found in a large range of different bioactive compounds. Moreover, gram scale reactions, as well as the application of suitable product transformations to natural products and key intermediates thereof are demonstrated.
D. Berthold, A. G. A. Geissler, S. Giofré, B. Breit, Angew. Chem. 2019, 131, 10099-10102. (link) Angew. Chem. Int. Ed. 2019, 58, 9994-9997. (link)
New Publication in Angewandte Chemie
Rhodium-Catalyzed Parallel Kinetic Resolution of Racemic Internal Allenes Towards Enantiopure Allylic 1,3-Diketones
A rare case of a parallel kinetic resolution of racemic 1,3‐disubstituted allenes by means of a rhodium‐catalyzed addition to 1,3‐diketones furnishing enantiopure allylic 1,3‐diketones is described. Mechanistic experiments demonstrate that the different allene enantiomers react in parallel to either the diastereomeric E‐ or Z‐allylic 1,3‐diketones with the same absolute configuration of the newly formed stereogenic center. A broad substrate scope demonstrates the synthetic utility of this new method.
L. Hilpert, B. Breit, Angew. Chem. 2019, 131, 10044-10048. (link) Angew. Chem. Int. Ed. 2019, 58, 9939-9943. (link)
New Publication in Chemical Science
A Rhodium-Catalyzed Cycloisomerization and Tandem Diels-Alder Reaction for Facile Access to Diverse Bicyclic and Tricyclic Heterocycles
A regioselective distal cycloisomerization of 1,6-allenenes was successfully developed to afford six-membered ring exocyclic 1,3-dienes employing a rhodium/diphosphine catalyst system. Deuterium labelling experiments and DFT calculations were performed to provide insights into the reaction mechanism of this unprecedented transformation. In addition, one-pot tandem Diels–Alder reactions with various dienophiles could readily construct diverse bicyclic and tricyclic nitrogen heterocycles, which are ubiquitous core scaffolds for a variety of natural products and bioactives. High efficiency and exclusive chemo and regioselectivities for a broad substrate scope were achieved under mild conditions using a low catalyst loading of 0.5 mol%.
Y. Zhou, A. Nikbakht, F. Bauer, B. Breit, Chem. Sci. 2019, 10, 4805-4810. (link)
New Publication in Chemical Science
Transition Metal Catalyzed Synthesis of syn- and anti-delta-Vinyl-Lactames: Formal Total Synthesis of (-)-Cermizine C and (-)-Senepodine G
A stereodivergent and diastereoselective transition-metal-catalyzed intramolecular hydroamidation of allenes and alkynes furnishing delta-vinyl-lactams is reported. Employing a rhodium catalyst allowed for the selective synthesis of the syn-delta-lactam. Conversely, a palladium catalyst led to the formation of the anti-delta-lactam in high selectivity. The new method shows high functional group compatibility and assorted synthetic transformations were demonstrated as well as its utility for the enantioselective formal total syntheses of (−)-cermizine C and (−)-senepodine G.
J. P. Schmidt, B. Breit, Chem. Sci. 2019, 10, 3074-3079. (link)
New Publication in Angewandte Chemie
Regiodivergent Hydroaminoalkylation of Alkynes and Allenes by a Combined Rhodium and Photoredox Catalytic System
A direct cross‐coupling of alkynes and allenes with amines to access α‐allylated amines using a Rh/photoredox dual catalyst system was developed. Starting from easily available internal alkynes, tertiary amines, and secondary amines, various branched homoallylic amines were obtained with good to excellent yields and regioselectivity. In contrast, with a modified reaction conditions, the alkynes and terminal allenes could couple with various substituted N‐aryl‐tetrahydroisoquinolines smoothly affording (E/em)‐linear homoallylic amines in good to excellent yields and regioselectivity.
J. Zheng, B. Breit, Angew. Chem. 2019, 131, 3430-3435; (link) Angew. Chem. Int. Ed. 2019, 58, 3392-3397. (link)
New Publication in Angewandte Chemie
Palladium- and Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes Towards Chiral Pyrazole
A complementing Pd‐ and Rh‐catalyzed dynamic kinetic resolution (DKR) of racemic allenes leading to N‐allylated pyrazoles is described. Such compounds are of enormous interest in medicinal chemistry as drugs and drug candidates. The new methods feature high chemo‐, regio‐ and enantioselectivity displaying a broad substrate scope and functional group compatibility. A mechanistic rational explanation for allene racemization and trans‐alkene selectivity is discussed.
L. J. Hilpert, S. V. Sieger, A. M. Haydl, B. Breit, Angew. Chem. 2019, 131, 3416-3419; (link) Angew. Chem. Int. Ed. 2019, 58, 3378-3381. (link)
New Publication in Angewandte Chemie
Tandem Regioselective Hydroformylation‐Hydrogenation of Internal Alkynes Using a Supramolecular Catalys
Good behaviour: A supramolecular catalyst enables a tandem Rh‐catalysed hydroformylation‐hydrogenation of unsymmetrical internal alkynes, functionalized with carboxylic acids in the β‐position, to give access to aliphatic aldehydes in high regio‐ and chemoselectivities. Control experiments confirm the enzyme‐like catalyst behaviour. DCE=1,2‐dichloroethane, CSA=camphorsulfonic acid.
W. Fang, B. Breit, Angew. Chem. 2018, 130, 15033-15037; (link) Angew. Chem. Int. Ed. 2018, 57,14817-14821. (link)
New Publication in Angewandte Chemie
Enantioselective Rhodium‐Catalyzed Dimerization of ω‐Allenyl Carboxylic Acids: Straightforward Synthesis of C2‐Symmetric Macrodiolides
Two in one: A highly atom‐efficient one‐step rhodium‐catalyzed dimerization of ω‐allenyl carboxylic acids was developed that furnishes C2‐symmetrical homodiolides, a structural motif found in numerous natural products. The method features high enantioselectivity, generating two stereogenic centers concomitantly.
P. Steib, B. Breit, Angew. Chem. 2018, 130, , 6682-6686; (link) Angew. Chem. Int. Ed. 2018, 57,6572-6576. (link)
New Publication in Angewandte Chemie
Inducing Axial Chirality in a Supramolecular Catalyst
Self‐organization of two chiral ligands coordinated to a metal center and connected by a twisted, hydrogen‐bonded backbone gives axially chiral supramolecular complexes. Formation of the tropos diastereomers was studied by a combination of computational and spectroscopic methods. The self‐assembled ligand systems were evaluated in rhodium‐catalyzed asymmetric hydrogenation and led to high product enantioselectivities.
K. M. Wenz, G. Leonhardt‐Lutterbeck, B. Breit, Angew. Chem. 2018, 130, 5194-5198 ; (link) Angew. Chem. Int. Ed. 2018, 57,5100-5104. (link)
Open House - AK Prof. Breit (Achtung, Raum- und Zeitänderung)
Bei der Vorstellung der Bachelorthemen des AK Breit gibt es eine Termin- und Raumänderung. Die Open House Veranstaltung findet nun am Freitag, 25.01.19 um 12:15 Uhr im SR 04002 statt.
New Publications in Angewandte Chemie
Rhodium-Catalyzed Regioselective Domino Azlactone-Alkyne Coupling/Aza-Cope Rearrangement: Facile Acess to 2-Allyl-3-oxazolin-5-ones and Trisubstituted Pyridines
A triple domino process involving in situ azlactone formation, rhodium-catalyzed alkyne coupling, and aza-Cope rearrangement furnishes useful 2-allyl-3-oxazolin-5-ones in one step. Thermolysis of these products results in pyridines, thereby providing a facile approach to de novo pyridine synthesis.
J. Kuang, S. Parveen, B. Breit, Angew. Chem. 2017, 129, 8542-8545 ; (link) Angew. Chem. Int. Ed. 2017, 56, 8422-8425. (link)
New Publications in Angewandte Chemie
Regio- and Enantioselective Rhodium-Catalyzed Addition of 1,3-Diketones to Allenes: Construction of Asymmetric Tertiary and Quaternary All Carbon Centers
Rh-Catalysis: A regio- and enantioselective atom-economic catalytic addition of 1,3-diketones to allenes is reported furnishing tertiary and quaternary all-carbon stereocenters under mild reaction conditions. The reaction shows a broad functional-group tolerance and numerous variations on both reaction partners highlight its synthetic utility.
T. M. Beck, B. Breit, Angew. Chem. 2017, 129, 1929-1933 ; (link) Angew. Chem. Int. Ed. 2017, 56, 1903-1907. (link)
Enantioselective and Regiodivergent Addition of Purines to Terminal Allenes: Synthesis of Abacavir
An atom-economic and regiodivergent Rh- and Pd-catalyzed coupling of purine derivatives and terminal allenes has been developed. High regioselectivity and excellent enantiomeric excess and yields were achieved with various functionalized substrates. Additionally, the developed methodology was applied to a straightforward synthesis of carbocyclic nucleoside abacavir.
N. Thieme, B. Breit, Angew. Chem. 2017, 129, 1542-1546 ; (link) Angew. Chem. Int. Ed. 2017, 56, 1520-1524. (link)
New Publication in Angewandte Chemie
Rhodium-Catalyzed Diastereoselective Cyclization of Allenyl-Sulfonylcarbamates: A Stereodivergent Approach to 1,3-Aminoalcohol Derivatives
A diastereoselective and stereodivergent rhodium-catalyzed intramolecular coupling of sulfonylcarbamates with terminal allenes is described and it provides selective access to 1,3-aminoalcohol derivatives, scaffolds found in bioactive compounds. The reaction is compatible with a large range of different functional groups, thus furnishing products with high diastereoselectivities and yields. Moreover, multigram scale reactions, as well as the application of suitable product transformations were demonstrated.
P. Spreider, A. Haydl, M. Heinrich, B. Breit Angew. Chem. 2016, 128, 15798-15802 ; (link) Angew. Chem. Int. Ed. 2015, 55, 15569-15573. (link)
New Publications in Angewandte Chemie and Accounts of Chemical Research
Rhodium-Catalyzed Enantioselective Intermolecular Hydroalkoxylation of Allenes and Alkynes with Alcohols: Synthesis of Branched Allylic Ethers
Regio- and enantioselective additions of alcohols to either terminal allenes or internal alkynes provides access to allylic ethers by using a RhI/diphenyl phosphate catalytic system. This method provides an atom-economic way to obtain chiral aliphatic and aryl allylic ethers in moderate to good yield with good to excellent enantioselectivities
Z. Liu, B. Breit, Angew. Chem. 2016, 128, 8580–8583 ; (link) Angew. Chem. Int. Ed. 2016, 55, 8440-8443. (link)
Enantioselective Rhodium-Catalyzed Atom-Economical Macrolactonization
A highly attractive route toward macrolactones, which form the cyclic scaffold of a multitude of diverse natural compounds, is described. Although many chemical approaches to this structural motif have been explored, an asymmetric variant of the cyclization is unprecedented. Herein we present an enantioselective macrolactonization through an intramolecular atom-economical rhodium-catalyzed coupling of ω-allenyl-substituted carboxylic acids. The use of a modified diop ligand, chiral DTBM-diop, led to high enantioselectivity (up to 93 % ee). The reaction tolerated a large variety of functionalities, including α,β-unsaturated carboxylic acids and depsipeptides, and provided the desired macrocycles with very high enantio- and diastereoselectivity.
S. Ganss, B. Breit, Angew. Chem. 2016, 128, 9890-9894 ; (link) Angew. Chem. Int. Ed. 2015, 55, 9738-9742. (link)
Branching Out: Rhodium-Catalyzed Allylation with Alkynes and Allenes
We present a new and efficient strategy for the atom-economic transformation of both alkynes and allenes to allylic functionalized structures via a Rh-catalyzed isomerization/addition reaction which has been developed in our working group. Our methodology thus grants access to an important structural class valued in modern organic chemistry for both its versatility for further functionalization and the potential for asymmetric synthesis with the construction of a new stereogenic center. This new methodology, inspired by mechanistic investigations by Werner in the late 1980s and based on preliminary work by Yamamoto and Trost, offers an attractive alternative to other established methods for allylic functionalization such as allylic substitution or allylic oxidation. The main advantage of our methodology consists of the inherent atom economy in comparison to allylic oxidation or substitution, which both produce stoichiometric amounts of waste and, in case of the substitution reaction, require prefunctionalization of the starting material. Starting out with the discovery of a highly branched-selective coupling reaction of carboxylic acids with terminal alkynes using a Rh(I)/DPEphos complex as the catalyst system, over the past 5 years we were able to continuously expand upon this chemistry, introducing various (pro)nucleophiles for the selective C–O, C–S, C–N, and C–C functionalization of both alkynes and the double-bond isomeric allenes by choosing the appropriate rhodium/bidentate phosphine catalyst. Thus, valuable compounds such as branched allylic ethers, sulfones, amines, or γ,δ-unsaturated ketones were successfully synthesized in high yields and with a broad substrate scope. Beyond the branched selectivity inherent to rhodium, many of the presented methodologies display additional degrees of selectivity in regard to regio-, diastereo-, and enantioselective transformations, with one example even proceeding via a dynamic kinetic resolution. Many advances presented in this account were driven by detailed mechanistic investigations including DFT-calculations, ESI-MS and in situ IR experiments and enabled the application of our chemistry for target-oriented syntheses demonstrated by several examples shown herein. In general, this research topic has matured over the past years into a viable option when synthesizing chiral compounds, from small molecules such as quercus lactones to complex target structures such as Homolargazole or Clavosolide A. This demonstrates the importance and utility of these coupling reactions, especially considering the ease with which carbon–heteroatom bonds can be built stereoselectively, with many of the product classes displaying motifs common in modern APIs.
P. Koschker, B. Breit, Acc. Chem. Res. 2016, 49, 1524-1536 ; (link)
New Publication in Angewandte Chemie
Stereodivergent and Protecting-Group-Free Synthesis of the Helicascolide Family: A Rhodium-Catalyzed Atom-Economical Lactonization Strategy
All in the family: The natural product family of the helicascolides A–C are one of countless groups of natural products containing six-membered lactones in their core structure. The rhodium-catalyzed regio- and diastereoselective addition of carboxylic acids with allenes permits the atom-economic and highly diastereoselective synthesis of the lactone core and allows for rapid access to this product family.
A. Haydl, D. Berthold, P. A. Spreider, B. Breit, Angew. Chem. 2016, 128, 5859–5863; (link) Angew. Chem. Int. Ed. 2016, 55, 5765–5769. (link)
New Publications in Angewandte Chemie and Chemical Science
Atom-Economical Dimerization Strategy by the Rhodium-Catalyzed Addition of Carboxylic Acids to Allenes: Protecting-Group-Free Synthesis of Clavosolide A and Late-Stage Modification
Better late than early: The natural product clavosolide A features a C2-symmetric core. A rhodium-catalyzed dimerization reaction involving the regio- and diastereoselective addition of carboxylic acids to allenes (see scheme) provided rapid access to this complex structure in only eight steps from penta-3,4-dienal and a readily accessible chiral crotyl-transfer reagent. The method is broadly applicable and suited to late-stage diversification.
A. Haydl, B. Breit, Angew. Chem. 2015, 127, 15750–15754; (link) Angew. Chem. Int. Ed. 2015, 54, 15530–15534. (link)
Z-Selective Hydrothiolation of Racemic 1,3-Disubstituted Allenes: An Atom-Economic Rhodium-Catalyzed Dynamic Kinetic Resolution
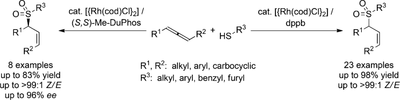
Zelectivity: The title reaction permits the synthesis of valuable allylic thioethers and sulfones in excellent Z selectivity. By using unsymmetrically 1,3-disubstituted allenes, good to high regioselectivities were obtained. Asymmetric hydrothiolation of racemic allenes with (S,S)-Me-DuPhos meets the criteria of a dynamic kinetic resolution. Me-DuPhos=2′,5′,2′′,5′′-tetramethyl-1,2-bis(phospholanyl)benzene.
A. B. Pritzius, B. Breit, Angew. Chem. 2015, 127, 16044–16048; (link) Angew. Chem. Int. Ed. 2015, 54, 15818–15822. (link)
Asymmetric synthesis of allylic amines via hydroamination of allenes with benzophenone imine
Rhodium-catalyzed highly regio- and enantioselective hydroamination of allenes is reported. Exclusive branched selectivities and excellent enantioselectivities were achieved applying a rhodium(I)/Josiphos catalyst. This method permits the practical synthesis of valuable α-chiral allylic amines using benzophenone imine as ammonia carrier.
K. Xu, Y.-H. Wang, V. Khakyzadeh, Chem. Sci. 2016, 7, 3313-3316; (link)
Khwarizmi International Award for Prof. Dr. Bernhard Breit
The Iranian Research Organization for Science and Technology (IROST), affiliated to the Iranian Ministry of Science, Research and Technology, is awarding the Khwarizmi International Award to Prof. Breit for his dedication, excellence and sustained hard work in the field of research. Since 1987, the KIA is presented to scientists for outstanding achievements and innovations that advance science and technology. It is the most important Iranian distinction of its kind and is presented by the President of Iran, Hassan Rouhani, and the Iranian Minister for Science, Research and Technology.
The award ceremony took place on 7 March 2016 in Iran’s capital Tehran in the presence of Prof. Breit. He is staying in Iran for several days and will present his research at renowned Iranian universities and institutions.
Sustainable catalysts: a German-Japanese cooperation combines the strong points of the University of Freiburg and expertise from Nagoya
The project “Multicomponent Supramolecular Catalysts for Sustainable Chemical Synthesis” conducted by Prof. Dr. Bernhard Breit (University of Freiburg), Prof. Dr. Takashi Ooi and Prof. Dr. Kenichiro Itami (Nagoya University) tackles the development of environmentally friendly and energy-efficient catalysts. Catalysts are substances that increase the rate of a chemical reaction by minimizing the required amount of activation energy, an energetic barrier between chemical reaction partners. During this process, the catalysts themselves are not consumed.
Around 80 percent of all chemical products are manufactured using catalytic processes. This means that increased environmental compatibility and energy efficiency of catalysts entails direct positive effects in the manufacture of chemical and pharmaceutical products. The project group draws inspiration from natural catalysts, such as enzymes, and aims to develop a new generation of supramolecular catalysts. Furthermore, the group’s groundbreaking research is to lay the basis for an international graduate school.
New Postdoctoral position in Organic Synthesis/Medicinal Chemistry
The research group of Prof. Breit at the Institute for Organic Chemistry is offering a
Postdoctoral position in Organic Synthesis/Medicinal Chemistry
At the Institute of Organic Chemistry at the Albert-Ludwigs University Freiburg in the research group of Professor Breit a postdoctoral position is available. The position is sponsored by the collaborative research center “Medical Epigenetics - SFB992“ and is part of a team consisting of structural biology and bioinformatics. The scientific goals are to synthesize small target molecules (primarily heterocyclic structures) and to evaluate within this team the biological properties, binding properties towards epigenetic target proteins in order to identify new molecular probes. Extensive experience in preparative organic chemistry including multistep synthesis is expected.
Please send a letter of motivation, a complete CV, a summary of research achievements and two letters of recommendation to Prof. Dr. Bernhard Breit.
New Publication in Nature Communications
Asymmetric synthesis of N-allylic indoles via regio- and enantioselective allylation of aryl hydrazines
The asymmetric synthesis of N-allylic indoles is important for natural product synthesis and pharmaceutical research. The regio- and enantioselective N-allylation of indoles is a true challenge due to the favourable C3-allylation. We develop here a new strategy to the asymmetric synthesis of N-allylic indoles via rhodium-catalysed N-selective coupling of aryl hydrazines with allenes followed by Fischer indolization. The exclusive N-selectivities and good to excellent enantioselectivities are achieved applying a rhodium(I)/DTBM-Segphos or rhodium(I)/DTBM-Binap catalyst. This method permits the practical synthesis of valuable chiral N-allylated indoles, and avoids the N- or C-selectivity issue.
K. Xu, T. Gilles, B. Breit, Nat. Commun., 2015, 6, 7616; (link)